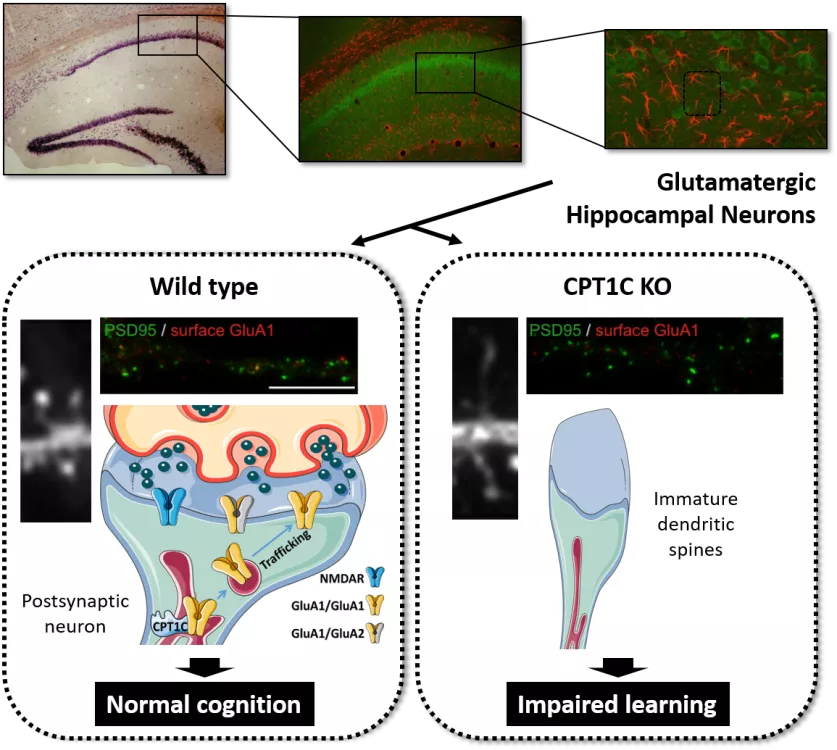
During learning and memory processes, external stimuli produce continuous adjustments in the synaptic strength of the glutamatergic neural networks of the hippocampus. This phenomenon, called synaptic plasticity, is tightly regulated by the number and the function of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) at the postsynaptic level. AMPARs mediate most of the fast excitatory transmission in the central nervous system. Its intracellular trafficking, from different organelles (such as the endoplasmic reticulum, Golgi apparatus and endosomal vesicles) to the plasma membrane is strongly regulated by a wide range of proteins. In turn, dysregulation of AMPAR trafficking is linked to most neurological and neurodegenerative disorders, including Alzheimer's disease and cognitive aging.
The dynamic behaviour of AMPA receptors is also regulated by different metabolic situations. In fact, hormones such as ghrelin (with an orexigenic effect: hunger sensation) or leptin (with an anorexic effect: satiety) promote synaptic incorporation of AMPARs, memory and learning. In contrast, fasting situations, high-fat diets or metabolic diseases impair glutamate transmission, hippocampal synaptic plasticity and cognition. However, the molecular mechanisms underlying metabolic control of these processes have not been fully identified. Interestingly, high-resolution proteomic analyses have revealed that one of the constituents of the complex of (AMPARs) is carnitine palmitoyltransferase 1C (CPT1C). CPT1s are enzymes that are usually involved in long-chain fatty acid transport across mitochondrial membrane. However, the brain-specific isoform CPT1C exhibits low catalytic activity in vitro and is located in the endoplasmic reticulum of neurons, largely in the hippocampus. Previous results in our laboratory demonstrated the involvement of hippocampal CPT1C in cognition and spinogenesis. CPT1C knockout (KO) mice were shown to present strongly compromised spatial learning and disrupted dendritic spine morphology by increasing immature filopodia and decreasing mature spine density. Moreover, we newly identify CPT1C as a regulator of AMPAR translation efficiency and therefore also synaptic transmission in the hippocampus. Additionally, other studies have described the involvement of CPT1C in GluA1 trafficking.
We are currently studying whether CPT1C could be regulating synaptic levels of AMPAR under different metabolic situations. Using biochemical and molecular approaches, we want to explore whether CPT1C, through its binding to malonyl-CoA, a precursor in the synthesis of fatty acids, could be sensing the neuronal energy state and, consequently, controlling the trafficking of AMPA receptors to the plasma membrane.